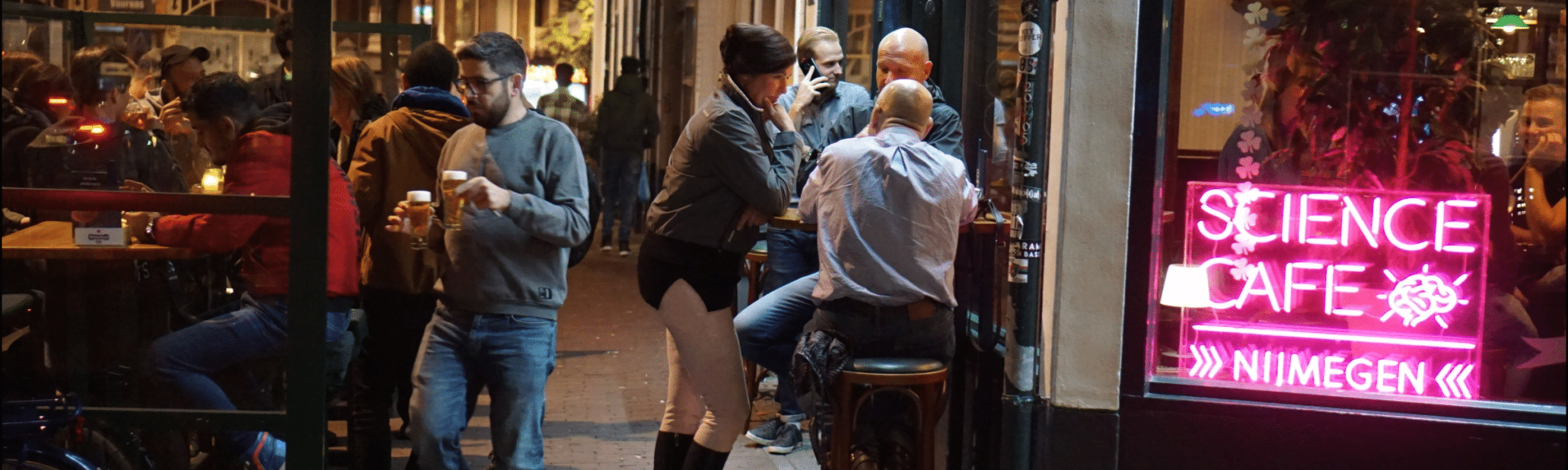Invite Meet&Eat 1 July 2025
Join the Meet&Eat (Residents only) July 1st, 2025 12:00 – 13:00 @Noviotech Campus, Pop-up event venue We warmly invite all...Noviotech Campus Soccer Tournament 2025
It's going to happen again, Friday afternoon, June 20, 2025, the "Noviotech Soccer Tournament" will take place on Quick's fields!...Invite walk-in pop-up tent (exclusively for Noviotech Campus employees)
On Thursday 12 June, we’re hosting our second informal get-together exclusively for Noviotech Campus employees. You're welcome to drop by...Meeting Your Peers Engineers #5 The Evolution of Sinter and Mold technology with Boschman
Date: June 10th, 2025 Time: 12:00 – 13:45 (Lunch included) Location: Noviotech Campus, Building M, Meet&Greet (Lunch in pop-up venue)...Beginners Training 7-Hills Run
Want to improve your running skills in a fun and accessible way—and get ready for the Zevenheuvelenloop? Starting at the…
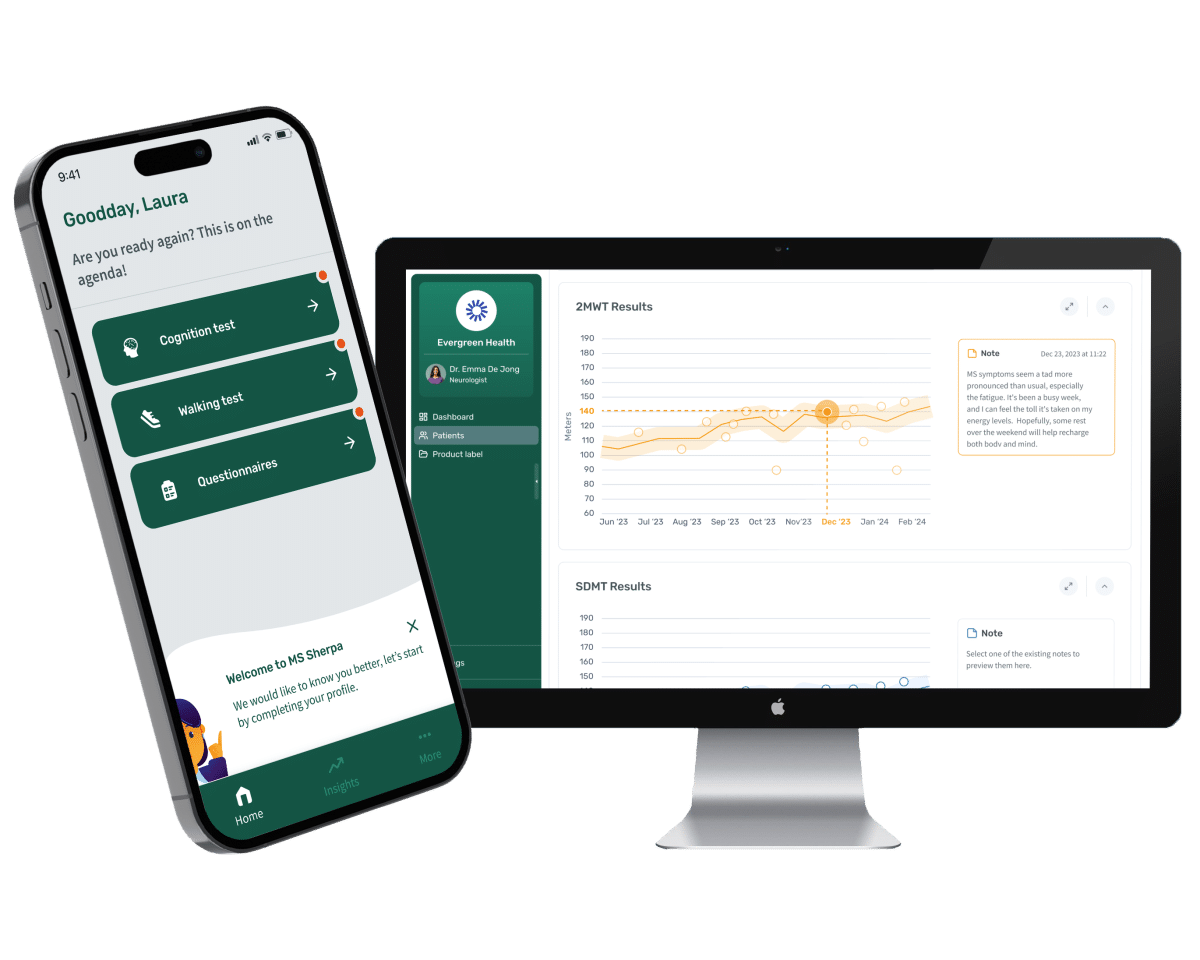
Invite Meet&Eat I Discover MS Sherpa I 3 June (registration closed)
Join the Meet&Eat (Residents only) June 3th, 2025 12:00 – 13:00 @Noviotech Campus, Pop-up event venue We warmly invite you...Dag van Winkelsteeg op Noviotech Campus 20 mei 2025
Kom kijken naar de toekomst van Winkelsteeg Op dinsdag 20 mei 2025 organiseren we de 'Dag van Winkelsteeg'. Je bent...Officiële start bouw Frontrunner op Noviotech Campus 14 mei 2025
Deze bijeenkomst is exclusief voor genodigden en alle campus bewoners. Op woensdag 14 mei 2025 markeren we een bijzonder moment...Invite Meet&Eat Pitches Start-ups Mercator Launch 6 May 2025
Join the Meet&Eat (Residents only) May 6th, 2025 12:00 – 13:00 @Noviotech Campus We warmly invite you to our monthly...Meet&Eat – Get to know RCT Gelderland April 8th 2025
Join the Meet&Eat April 8th, 2025 12:00 – 13:00 @Noviotech Campus (Building M) We warmly invite you to the next...Webinar: Business opportunities in Arizona and highlight key industries
Hi there! Did you know that Phoenix is one of the fastest growing cities in the US for business expansion?...Meeting Your Peers Engineers #4 – NXP Semiconductors: High thermal die attach materials: their performance and reliability in semiconductor packages 18 March 2025
Date: March 18th, 2025 Time: 12:00 – 13:45 (Lunch included) Location: Noviotech Campus, Building M, Meet&Greet We invite you to...Meeting Your Peers: HR #5 I Theme: Lifeport Chip Academy/Beethoven
We are pleased to invite all HR professionals working at companies on Noviotech Campus to the next "Meeting Your Peers...Meet&Eat – Get to know Sencure March 11th 2025
Meet&Eat - Get to know Sencure Join the Meet&Eat March 11th, 2025 12:00 – 13:00 @Noviotech Campus (Building M) We...Meeting Your Peers: Marcom & Office #6
It's that time again! The Marketing, Communication & Office professionals working at one of the companies on Noviotech Campus are...Meet&Eat – GATT Technologies February 4th 2025
Meet&Eat - GATT Technologies We are excited to invite you to a Meet & Greet session, where Plant Manager Agnieszka...Meeting Your Peers: HR #4 Attracting and retaining talent
We are excited to invite you to the next "Meeting Your Peers HR" event on January 16, 2025 – a...Meet & Eat Sint Edition – 3 Dec 2024
Meet & Eat 3 December 2024 SINT EDITION @Building M Noviotech Campus, Nijmegen Come and celebrate Sinterklaas during the special edition...Meeting Your Peers Engineers #3 – ITEC: Redefining Semiconductor Manufacturing – 28 Nov 2024
We’re organizing the 3rd Meeting Your Peers Engineers lunch event on Thursday November 28 at Noviotech Campus. Engineers from semiconductor...Winkelsteeg Fun Run
On Thursday 14 November, the municipality of Nijmegen is organising the Winkelsteeg Fun Run from 14.30 to 17.30. This is...Meet&Eat on tour – EXPLORING NEXPERIA (Fully Booked)
Meet&Eat on tour - EXPLORING NEXPERIA (Registration is CLOSED: event is full) We are excited to invite you to the...Meeting Your Peers: HR #3 Terms of Employment
Do you work in the HR department of a company located on the Noviotech Campus? Then join this meeting! We...Meeting Your Peers: Marcom & Office #6
It's that time again! The Marketing, Communication & Office professionals working at one of the companies on Noviotech Campus are...Roadshow Linde Gas Cryoservices
As part of our roadshow we will visit the Noviotech Campus on October the 3rd. The theme of the roadshow...Meet&Eat on tour – EXPLORING NTS (Registration closed)
''Meet&Eat on tour October 1st 2024'' EXPLORING NTS We warmly invite you to our monthly Meet&Eat on October 1st from...Meeting Your Peers Engineers #2 – Sustainability in Chip Packaging – Sep 26th (Registration closed)
How is sustainability applied in Chip Packaging? (Registration closed) By popular demand, the High Tech Network Meetings are back in...INNOVATE Meetup
Innovate Meetup Explore • Connect • Innovate Organizing during the Vibe of the Future Festival It was an epic night...Walk&Talk
We’re excited to announce our very first Walk & Talk for our tenants! Join for a relaxed walk with fellow...SportUP: Volleybal edition
Take a break from your desk and join us for a fun SportUP activity! Starting this month, we will be...Meet&Eat: Pitches start-ups Mercator Launch
''Meet&Eat September 3th 2024'' PITCHES START-UPS MERCATOR LAUNCH We warmly invite you to our monthly Meet&Eat on September 3th from...Pitstops – 4Day March Nijmegen
Are you walking the 4-day? Let the Health & High Tech Pitstop take care of you along the way! The...Meet&Eat on tour – Exploring Pharma Delta
''Meet&Eat July 2th 2024'' EXPLORING PHARMA DELTA We warmly invite you to our monthly Meet&Eat on July 2th from 12:00...Meeting Your Peers: HR #2 Benefits & Mobiliteit
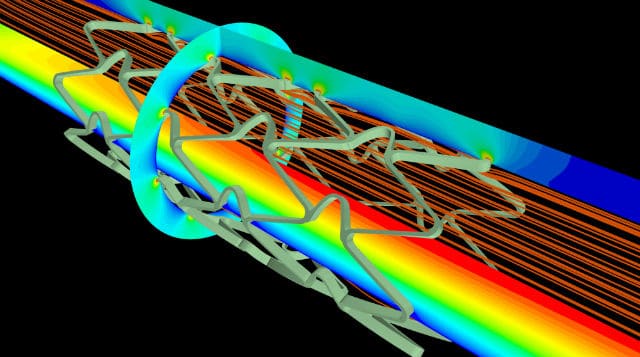
Do you work in the HR department of a company located on the Noviotech Campus? Then join this meeting! We...Meeting Your Peers Engineers #1 – Multiphysics Simulation – June 25th (Sold Out)
** SOLD OUT** We have good news! By popular demand, the High Tech Network Meetings are back in action. We...Meeting Your Peers: Marcom & Office #5
It's that time again! The Marketing, Communication & Office professionals working at one of the companies on Noviotech Campus are...Noviotech Soccer Tournament 2024
It's going to happen again, Friday afternoon, June 21, 2024, the "Noviotech Soccer Tournament" will take place on Quick's fields!...Opening Party Pop-up Horeca Noviotech Campus
On Thursday, June 20th, all residents of Noviotech Campus are invited to discover our brand-new Pop-up Horeca in the Green...Meet & Eat – Exploring Neways Electronics – 4 June 2024
”Meet & Eat 4 June 2024” EXPLORING NEWAYS ELECTRONICS @Building M, Noviotech Campus, Nijmegen We warmly invite you to our…
Benelux RF Conference 2024
𝗝𝗼𝗶𝗻 𝘁𝗵𝗲 𝗕𝗲𝗻𝗲𝗹𝘂𝘅 𝗥𝗙 𝗖𝗼𝗻𝗳𝗲𝗿𝗲𝗻𝗰𝗲 📅 29 May 2024 📍 Nijmegen, the Netherlands 🎤 Check the program on www.beneluxrf.com We’re...Meeting your Peers: Marcom & Office
Time to catch up and make new connections! We invite you to the Meeting Your Peers: Marcom & Office Event…
Meet&Eat on tour – EXPLORING EPR
''Meet&Eat on tour May 14th 2024'' EXPLORING EPR We warmly invite you to our monthly Meet&Eat on May 14th from...Meet&Eat – Human Capital April 2nd 2024
Theme of this ''Meet & Eat April 2nd 2024'' HUMAN CAPITAL We're hosting this Meet&Eat lunch meeting where we'll chat...Meeting Your Peers HR
We cordially invite you to the "Meeting Your Peers – HR" event with the theme: Collaborating in HR at Noviotech...Health Valley Event 2024
Transition in Healthcare The Health Valley Event on March 21, 2024, at Pathé Nijmegen marks its 15th anniversary edition as...InScience Film Festival
Excited for the next InScience Film Festival? Noviotech Campus is back as a partner for another rich program of films,...Meet&Eat – Sports & Vitality
Theme of this ''Meet & Eat March 5th 2024'' SPORTS & VITALITY Following the success from the Meet&Eat in April...Meet&Eat – Meet Students & Talents
Theme of this ''Meet&Eat February 6th 2024'' MEET STUDENTS & TALENTS After the success of the Meet&Eat in March 2023,...Christmas Charity for ALS
Visit our Christmas Charity for ALS Wednesday 20th Dec 15 -17 hrs @52Nijmegen End the year on a positive note...Meet & Eat – Special Sint Edition – 5 Dec 2023
Theme of this ''Meet & Eat 5 December 2023'' : SPECIAL SINT EDITION @Building M Noviotech Campus, Nijmegen Come and celebrate...Meeting your Peers (Marcom & Office)
Time to catch up and make new connections! Yvette and Marlou invite you to the ‘Meeting Your Peers’ event on…
Meet & Eat – Power of AI – 7 Nov 2023
Theme of this ''Meet & Eat 7 November 2023'' POWER OF AI @Building M, Noviotech Campus, Nijmegen The impact of...Radboud Art & Science
On October 17th 2023, we will celebrate the university’s official birthday: the 100th Dies Natalis. During the ceremony, we will…
Radboud 100 years celebration!
On October 17th, 2023, Radboud University, partner of Noviotech Campus, will turn 100 years old. On that date they will celebrate…
Meet & Eat event – Women in Tech – 3rd Oct 2023
Theme of this ''Meet & Eat 3 October 2023'' : WOMEN IN TECH In this fast-pacing world we need smart...Company Valuation & legal issues of joint ventures with investors
What’s the value of your company? Valuation of a company is almost always hard. Sentiments play a role, but also…
Parkinson weekend Nijmegen
During the weekend of September 22-24, the city of Nijmegen will be completely dedicated to Parkinson’s disease. Parkinson’s disease is…
Innoboot
Science is often inspirational, it shows the potential how we can improve society. But science in itself does not change...Groesbeeks Gruwelijkste
This is classic event must be on the bucket list of every Mountainbiker. “Gruwelijkste” means very challenging (up to…
Semicon Taiwan
Joint Silicon Europe / High Tech NL booth at SEMICON Taiwan 2023 SEMICON Taiwan is the most important annual trade…
1st EuroPAT-WS 2023
Save the Date: 6 & 7 Sept 2023 1st EuroPAT-WS 2023 – European Packaging, Assembly, and Test – Workshop 2023,…
Meet & Eat event – Welcome Back – 5th Sept 2023
Theme of this ''Meet & Eat 5 September 2023'' : WELCOME BACK from HOLIDAYS A big welcome back, and especially...Start Semiconductor Packaging University Program
Together with HAN University of Applied Sciences, CITC developed the Semiconductor Packaging University Program. The program provides a connection between education…
Special open-air Summer event
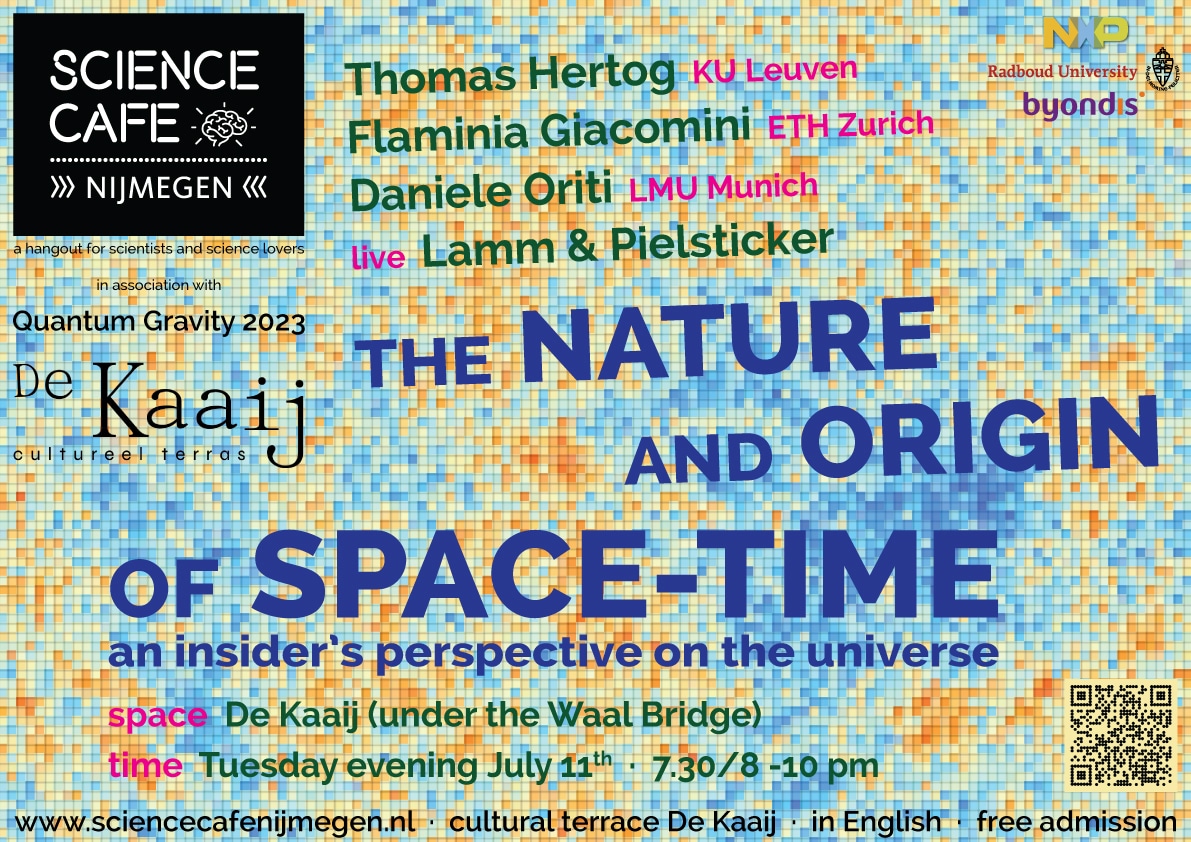
THE NATURE AND ORIGIN OF SPACE-TIME an insider’s perspective on the universe Would space and time exist if the universe...Workshop: How to get more out of your team?
Leading and managing a team is rewarding, complex, sometimes frustrating and amazing! Most entrepreneurs know more about their industry or…
Shiatsu Chair Massage
Delightful relaxing and energizing massage for treatment of back, neck, shoulders, arms and hands. Tailored to your body and needs.Book…
Business opportunities in the Philippines
Learn more about business opportunities in the Philippines in semiconductor, health and high tech sectors Visit Philippine embassy and minister…
#DareToAsk & BBQ
Nijmegen is the city of Health and High Tech with the vibe of the future! In our region we have many…
Lifeport Festival Circulair
Save the Date! Monday afternoon, June 26, for the Lifeport Festival Circular at the Radboudumc Experience Centre in Nijmegen. Regional…
Workshop: Hocus – Pocus – Focus
As an entrepreneur, do you also wish: * to be able to spend more time on important matters? * to…
Shiatsu
Delightful relaxing and energizing massage for treatment of back, neck, shoulders, arms and hands. Tailored to your body and needs.Book…
The Night of Space
Join us on a journey to the stars during this brand new edition of The Night of Space! Humanity has…
Pitch to Win
Practical tools to help you build your winning pitch. David Beckett Bio David Beckett is an international pitch coach, who…
Startup trade mission to London Tech Week 2023
Is your tech startup ready to expand internationally? Do you want to do business and meet up with companies or…
Tax aspects about hiring people: Risks and Opportunities
Join us for our workshop on Tax Aspects About Hiring People. Gain valuable insights into the tax implications of hiring…
Meet & Eat event – International – 6th June 2023
Theme of this ''Meet & Eat 6 June 2023'' : INTERNATIONAL Do you have any idea know how many different...Shiatsu Chair Massage
Delightful relaxing and energizing massage for treatment of back, neck, shoulders, arms and hands. Tailored to your body and needs….
Gravel tour in Maasduinen
Maasduinen is a Nature Reserve about 20 km South of Nijmegen. It’s ideal for Gravel biking with the many sandy…
Workshop: Business with impact! More business with a heart for society
The world is changing and that calls for more sustainable future-proof entrepreneurs. Whether you want to make impact with your…
The importance of solid general terms & conditions in contracts and agreements
Workshop Briskr& Poelmann van den BroekClear contractingDuring this interactive workshop Daniek and Jan Willem from our supporting partner Poelmann van…
From Molecule to Business event
Development of new medicines is a lengthy and laborious process which can take more than 10 years and can cost…
Benelux RF Conference 2023
Noviotech Campus is a proud sponsor of the Benelux RF Conference! Are you a community member, located at Noviotech Campus…
BBB Career event
On Wednesday 24th of May 2023, the BBB Career Event will take place! After the success of last year’s event,…
Workshop: behavior change
“Real influence is about understanding behavior. After all, when you understand behavior, you can change it.” Whether you want to…
Day of Business Parks
Are you looking for new business opportunities and possibilities to grow your business park? Then don’t miss the Day of…
Test Market Nijmegen for starting entrepeneurs
On May 21, 2023, in cooperation with the municipality of Nijmegen, we will organize the third edition of the Test…
10 steps to succesful CE marketing
Are you an innovative startup in the medical device industry and feel a bit overwhelmed by the Medical Devices Regulation…
Science Café: a Digital Democracy
“We are 21st-century citizens, doing our best to interact with 19th century-designed institutions that are based on an information technology…
Radboud Festival
Let’s celebrate! On 11 May, Radboud University will treat students, (former) staff, alumni and neighbours to a festival to celebrate its 100th anniversary. From live artists and DJs to inspiring speakers and enjoying the terrace with snacks and drinks. Will you be there?
Meet & Eat event – Inclusiveness & Diversity
Theme of this ''Meet & Eat 9 May 2023'' : Inclusiveness & Diversity Do you want to know why companies...Meeting of the HR-minds
Radboud University invites you to: ‘MEETING OF THE HR-MINDS’ at Lifeport Welcome Center (Exclusively for HR professionals in the Arnhem-Nijmegen…
The importance of solid general terms & conditions in contracts and agreements
Clear contracting During this interactive workshop Daniek and Jan Willem from our supporting partner Poelmann van den Broek advocaten will…
PCIM Europe exhibition
From May 9 – 11, some companies like CITC will participate in PCIM Europe, the leading international exhibition and conference…
100 Radboud Gestures
In the week of 8 to 14 May, our partner Radboud University will surprise Nijmegen and its inhabitants with 100…
Noviotech Climbing Week
7 - 13 May 2023 - In this week you can join groups or ride individual and you will ride...Workshop: Business vlogging with your smartphone
Most people know vlogging as a peek into someone’s daily life. Nowadays, the business world has also taken to vlogging,…
FuckUp Night by StartUP Nijmegen
From failures to successes: learn from the mistakes of experienced entrepreneurs during FuckUp Night. Are you curious about the biggest…
Men’s or Women’s diseases
The significance of sex differences in biomedical research Traditionally, biomedical research has focused on the “70-kilogram man”. Differences between men’s…
Network event Master Molecular Life Sciences
Get inspired by science and professional speakers. Expand your network, share experiences, meet potential employers and employees Program 16.00: Walk-in…
SMB-meeting Patient driven Innovation
Healthcare is a peoples business. Keeping the patient not just in the center of the attention of innovation but also…
Deep Tech Atelier, Riga – Latvia
The Deep Tech Atelier @Riga will become a deep-tech hub for two days. The Baltics’ largest deep tech industry event brings…
Workshop: Using AI for your Startup
As an entrepreneur, of course you always want to be upfront of the latest developments. That is why we offer…
HAN workshop SEO
There is a lot involved in getting a top position in the Google results. Smart written website texts are of…
ION Knowledge Lunch
Have you already signed up for the ION Knowledge Lunch? We would like to bring this interactive and impactful meeting to…
How to attract venture capital investment for your innovation?
This workshop focuses on innovative startups/entrepreneurs that might not yet be ready to apply for series A capital but are...Regulatory Approaches in Novel Technologies and Methods
Regulatory approval can be a long and winding road, especially when submissions include novel technologies or innovative clinical trial setups….
Innovation for Health
On 6 April 2023 at WTC Rotterdam, the 10th edition of the leading conference for key players in Health &…
Meet & Eat event – Sport & Vitality 4 April 2023
Noviotech Campus is full of sportive opportunities! Curious to learn more about the various sports or want to increase your...Resilience – Learn how to bounce back from setbacks
As entrepreneurs we must be able to deal with different situations, positive and negative ones. We have to learn how…
NWO Physics Conference
After two online editions, NWO Physics is making a real-life comeback at NH Koningshof, Veldhoven! The 2023 edition of NWO…
HAN Workshop Planning & Timemanagement
Do you want better insight into tasks and actions, an overview of your planning and focus on the growth of…
Health Valley Event 2023
The Health Valley Event (HVE) on 30 March 2023 in Pathé Nijmegen is the leading healthcare innovation event in the…
Solar Fuels – out of air, water and sunlight
Simply obtaining fuels out of plain air, water and sunlight, wouldn’t that be awesome?! In fact, this is a reality…
Career Fair 2023
On Wednesday, March 23, the third edition of the BMW Career Fair will take place! Wondering exactly what your career…
Knowledge sharing event: Chip Design
An Industry Deep Dive: micro-electronics & heterogeneous systems. On March 23, some of the Netherlands’ leading experts in chip design…
HAN workshop Pitching with Passion
There are many times when it is to have a good pitch on hand. But How do you achieve maximum...How2Finance
How do I find the right financing for my business? For start-ups and growing entrepreneurs, financing is extremely important but…
CNRood & ELABO at Noviotech Campus
On Tuesday 21 March you can visit the demo van and discover the new ELABO Workstations at Noviotech Campus between…
InScience Festival
InScience, International Science Film Festival Nijmegen is one of the largest international science film festivals in Europe. The program consists…
Product development with customer validation
In their colorful studio in Helmond, Jeroen, founder of GBO Innovation Makers talks about the journey through the product development…
Networking and inspiration meeting
#kansvoornijmegen The theme of the meeting is “EXCELLEREN,” or showing what we are good at! Who better than our special…
Tech Meets Law Conference
On Wednesday 8 March, the TML Academy will host the first annual Tech Meets Law Congress. An afternoon full of…
Meet & Eat event – 7 March 2023
How challenges within your organizations could be the next internship, graduation project or student assignment. Together with Career Services* from...BANN meeting
The Business Angels Network Nijmegen, BANN network, is all about physical meetings, where investors can get to know each other…
HAN Workshop Online Marketing
ABOUT THE WORKSHOP How to take the right steps to achieve the above, you will learn in this workshop. The…
PSYCHOPATHY
Psychopathy has a public image of violence and crime, but research shows that this doesn't always have to be the...Innovation competition 2023 – Grande Finale
Do you want to be inspired by cool innovations or would like to meet fellow academic innovators? You are invited to…
Gelderse Circulaire Innovatie Top 20 @Noviotech Campus
Circular business is the future. The continuity of our manufacturing, construction, agriculture and many other sectors stands with the realization…
Meet & Eat event – 7 Feb 2023
Do you want to know more about the developments of the Noviotech Campus? Join the next Meet & Eat and...Masterclass AI for Business – Radboud Academy
DATE CHANGE from 29 Nov to 7 FEB 2023 On February 7, 2023, Radboud Academy is organizing the 1-day Masterclass…
Funding opportunities to speed up your innovation
During this interactive workshop you will learn key insights on private and public funding opportunities within the life science space…
Save the Date: Invitation Arnhem meets Duisburg
ENERGY, INNOVATION, LOGISTICS and STARTUPS The municipality of Arnhem and the Foundation German-Dutch Businessclub Gelderland organize a working visit to…
Reimbursement for your healthcare innovation
Who is your client? As an entrepreneur in healthcare you may have a great product to improve quality and efficiency…
Work smarter not harder!
Science Meet Business Network event The challenges on the healthcare labor market are huge, which is also noticeable in the…
Company Valuation & legal issues of joint ventures with investors
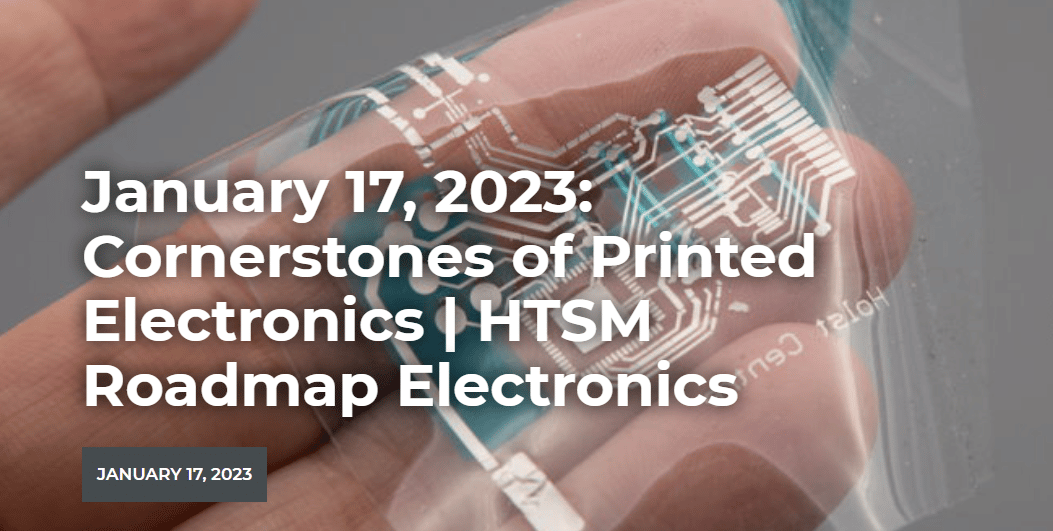
What’s the value of your company? Valuation of a company is almost always hard. Sentiments play a role, but also...Cornerstones of Printed Electronics
In collaboration with Printed Electronics Nederland and the HTSM Roadmap Electronics team we are organizing a meeting on printed electronics...Workshop: ”the psychology of influencing customers”
How do you convince others to become customers of yours become a customer of yours? How do you turn insta-followers...How do we remember (better)!
On January 16, 2023, memory experts Freyja Ólafsdóttir and Boris Konrad of the Radboud University will introduce us to the…
Follow-up session RF Technology lab at Radboud University
On Wednesday, January 11, 2023, the RF Knowledge Lab Follow-up session will take place. This meeting is hosted by Radboud…
Workshop ”more business with a heart for society”
The world is changing and is calling for more sustainable future-proof entrepreneurs. Whether you put impact ahead of your business…
The Swell of Spacetime
Space and time are not as we know them, a static backdrop in which our lives take place and the…
Better Together: Managing Term Sheets for a Successful Collaboration
For an early-stage life science company, collaboration with a large corporate partner may be critical to successfully bring an innovation…
Pitchen met Passie. Leer je bedrijf overtuigend te presenteren
Over de workshop Investeerder Shawn Harris zei volmondig “ja!” na de pitch van HAN-student Remco Hoftijzer in het programma Dragons’…
Meet & Eat – Sint edition
Do you want to meet Sinterklaas? This weekend, Sinterklaas traditionally arrived in the Netherlands for the big Sinterklaas party...Briskr workshop: Funding opportunities to speed up your innovation
During this interactive workshop you will learn key insights on private and public funding opportunities within the life science space…
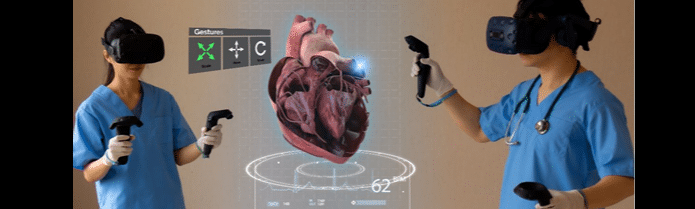
INNOVATE presents The Chip Generation
An evening on how chip technology answers to the challenges of our time, presented by Innovate, Nexperia, NXP & ITEC.Dutch Life Sciences Conference
Presents ”Innovations and new business strategies in Dutch Life Sciences” on 24th November. Don’t miss out and register. Please find…
Briskr workshop: Tax Opportunities for startups en scale-ups
Prepare yourself for the future by setting up your business structures smartly! That way you are more flexible and can…
Holland High Tech EVENT 2022
Let’s innovate together: sustainable & future-proof The Holland High Tech EVENT on 1 November is thé annual event where the...Schemerlight festival
Schemerlight Festival 2022 presents ”AIR SPACE The Netherlands’. This is an audiovisual arts festival for young & old. During the…
Workshop | Road to the Clinic for Small Molecules
Submitting a Clinical Trial Application is a critical early milestone for every biotech company. Join Johnson & Johnson Innovation –…
UNDERSTANDING COMPLEXITY
The all-encompassing view of complex adaptive systems. On Monday evening, October 17, the Science Café will host two leading scientists…
Cycletime Reichswald MTB tour
It is already becoming a tradition. Every year, in autumn NXP cycletime organizes the Reichswald MTB tour. This ride takes…
INNOVATE Energy Meetup
Together with program partner CONNECTR, we are providing an event on top entrepreneurship to achieve the 2030 climate goals. Expect…
The future of us – changing our ways!
The Future of Us! The informative festival takes place live at the Industrial Studios at IPKW. This year’s theme is…
Innovation Mission Semiconductors South Korea
Are you active in the semiconductor equipment industry and looking for Korean business or innovation partners? Then this innovation mission…
Open House
The Faculty of Science is celebrating its birthday and opens its doors. Celebrate the 65th birthday with our programme for…
Grand Opening building NTS Optel
On Friday 23th September 2022, Alderman of Economic Affairs John Brom officially opened the new building on the Novio Tech...The Science Café
the Science Café will bring together three experts, Tim Sweijs (HCSS), Ralph Savelsberg (NLDA) and Clara Maathuis (OU), to talk…
ART NIGHT 2022
Ready for something new? The Kunstnacht (Art Night) is a night full of unique experiences in which surprising combinations of…
The Night of Space
INNOVATE PRESENTS: The Night of Space What impact does space have on the challenges of our world? Innovators, scientists, artists…
The Vibe of the Future Festival 2022
www.vibeofthefuture.com
The Science Café
Kicks off the new season on September 4th! During the Fête de la Nature, for the first time in its…